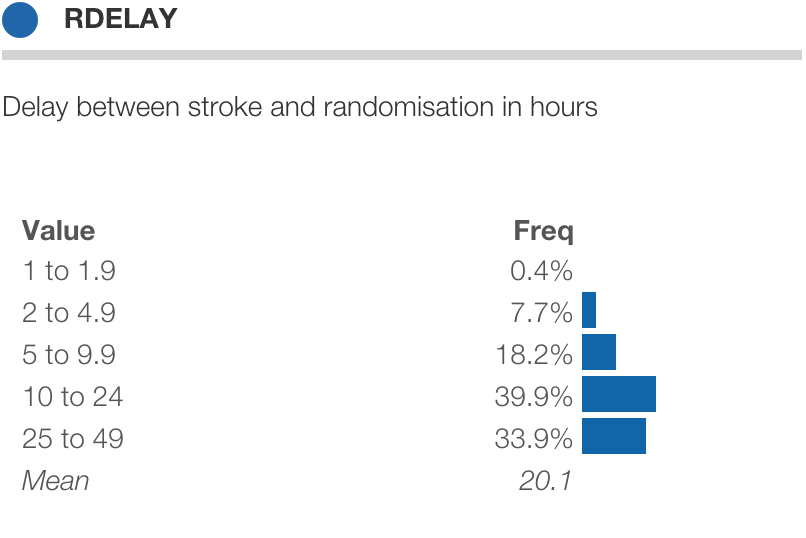
The International Stroke Trial (IST) was one the biggest randomised trials ever conducted in acute stroke. Patients were assessed at randomisation, at the early outcome point (14-days after randomisation or prior discharge) and at 6-months.
There is an incredible lack of transparency in clinical trial results today. A team at the University of Edinburgh has been working to change that, and has made the individual patient-level data available for public use, to facilitate the planning of future trials and to permit additional secondary analyses. Explore the data in Protobi.
This dataset contains information from International Stroke Trial database (version 2) which is made available under the ODC Attribution License. The full report is available here.
Sandercock, Peter; Niewada, Maciej; Czlonkowska, Anna. (2011). International Stroke Trial database (version 2), [Dataset]. University of Edinburgh, Department of Clinical Neurosciences.